Tag: generic drugs
The FD&C Act laid the groundwork for drug safety in the U.S., but the Hatch-Waxman Amendments created the legal path for generic drugs. Today, 90% of prescriptions are filled with generics thanks to this system.
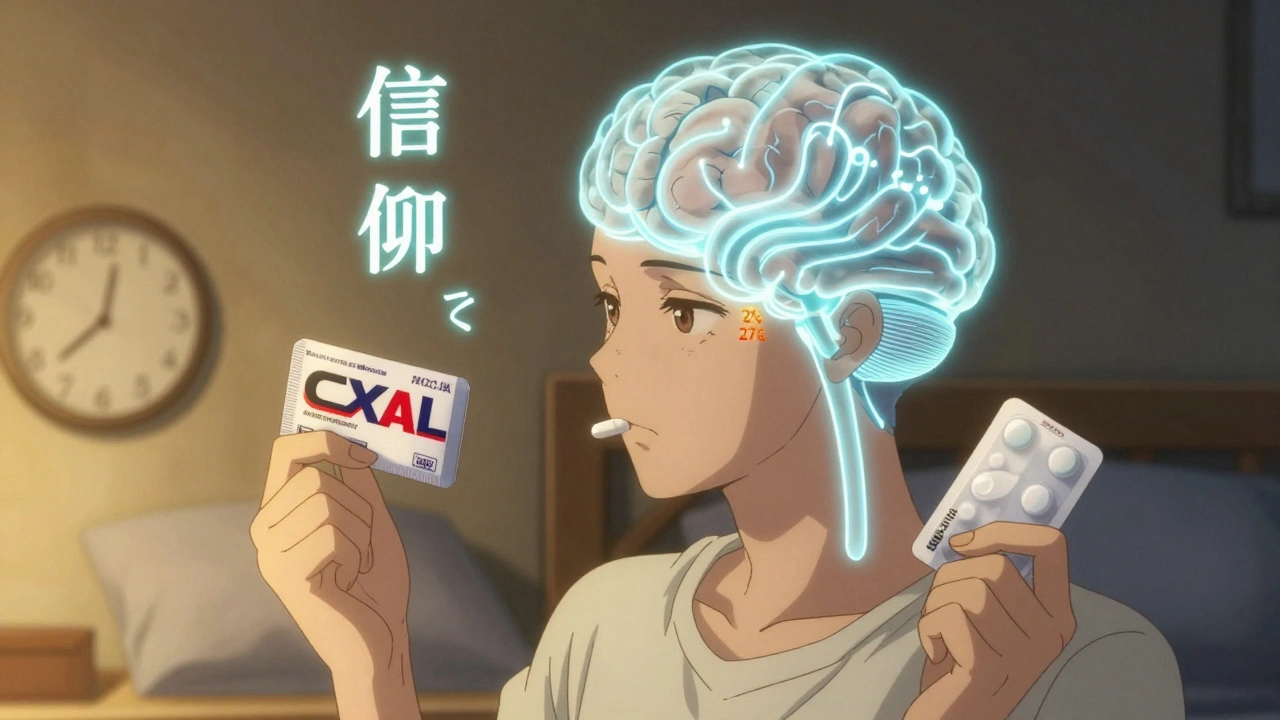
Generic drugs are chemically identical to brand-name versions, but many patients feel they don't work as well-because of the label. Learn how the labeling effect impacts pain, adherence, and treatment outcomes.
Track how your body responds when switching to generic medications with a simple medication journal. Learn what to record, why it matters, and how to use your data to work with your doctor.
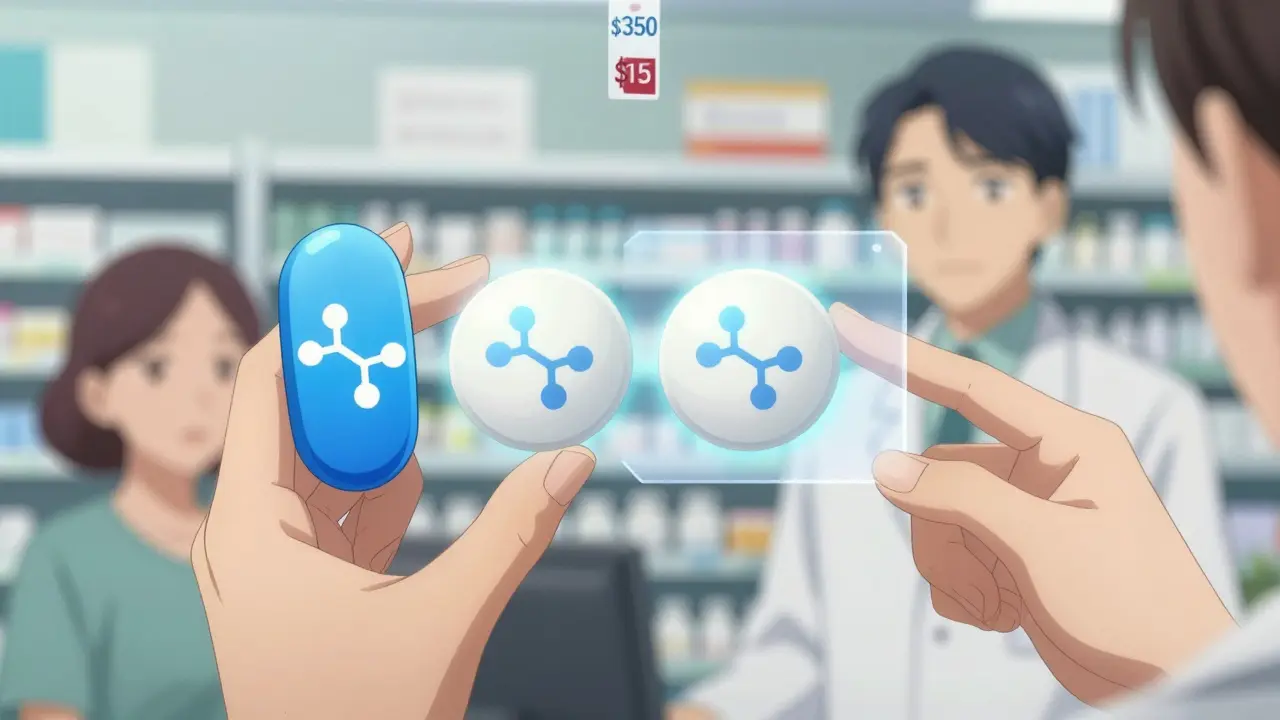
Switching from brand-name to generic medication can save you hundreds per month - and studies show it works just as well. Learn what to expect, when to be cautious, and how to make sure your treatment stays effective.
Despite making up 90% of prescriptions, generic drugs still face skepticism from many doctors. This article explores why providers hesitate, what data reveals about their attitudes, and how education and real-world evidence are changing prescribing habits.
Advertising for brand-name drugs influences patients to request expensive medications over equally effective generics, driving up costs without improving health outcomes. Here's how marketing shapes perceptions-and what you can do about it.
The Orange Book database is the FDA's official list of approved drugs with therapeutic equivalence ratings and patent information. It determines when generic drugs can enter the market, saving billions in healthcare costs.
Generic drugs are chemically identical to brand-name versions, but psychological factors like expectation and labeling can make them feel less effective. Learn how the placebo effect impacts adherence and what you can do about it.