When your pharmacist hands you a pill that looks nothing like the one you’ve been taking, it’s normal to feel uneasy. The color is different. The shape is odd. The name on the tablet is unfamiliar. You might wonder: Is this really the same thing? The short answer is yes - if it’s an FDA-approved generic. But there’s more to the story than just active ingredients. Knowing what to expect before, during, and after the switch can make all the difference in how you feel - and how much you pay.
Generics Aren’t Cheap Copies - They’re Required to Be the Same
Generic drugs aren’t knockoffs. They’re legally required to contain the exact same active ingredient, in the same strength, and delivered the same way as the brand-name version. If you’re taking metformin for diabetes, whether it’s called Glucophage or just "metformin," the molecule doing the work is identical. The FDA doesn’t allow generics to be "close enough." They must prove they work the same way in your body.
To get approval, generic manufacturers must show bioequivalence - meaning the drug gets into your bloodstream at the same rate and to the same level as the brand. The standard? Within 80% to 125% of the brand’s performance. That’s not a guess. It’s based on real blood tests in hundreds of volunteers. In fact, studies show that 88% of the time, generics perform just like the brand in clinical outcomes. A 2019 JAMA Internal Medicine review of over 2,000 trials found no meaningful difference in effectiveness or safety.
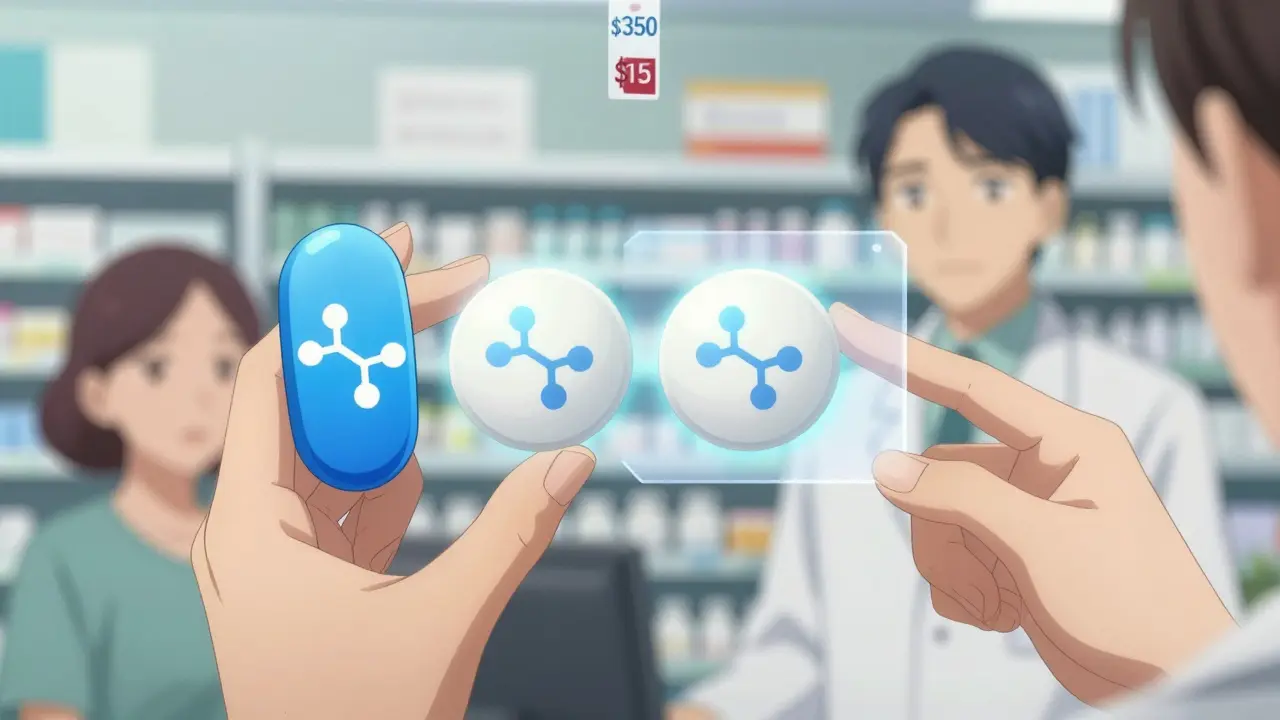
And the cost? That’s where the real difference shows up. Brand-name drugs can cost $600 or more per prescription. The generic version? Often under $20. On average, patients save 80% to 85% by switching. For someone on a monthly medication like lisinopril for high blood pressure, that could mean going from $350 to $15 a month. That’s not just savings - it’s access.
What You’ll Notice: Appearance, Not Effect
You’ll see the biggest change in how the pill looks. Generics can’t look like the brand-name version. That’s trademark law. So your blue oval pill might become a white round one. Your capsule might switch from red and white to clear and green. The size might be bigger or smaller. The imprint might say "M 20" instead of "Lipitor 20."
This change trips people up - especially older adults. One case reported to the FDA involved an 82-year-old woman who took two pills because she didn’t recognize the new generic version of her blood pressure med. She thought the old one was gone and the new one was a second dose. It’s not rare. Healthgrades data shows nearly 19% of negative reviews for generics cite confusion over appearance.
That’s why pharmacists are required to explain the switch. In most states, they give you a quick 5-7 minute chat when you pick up your first generic. They’ll point out the differences and remind you: "It’s the same medicine, just a different look."
When Generics Might Not Be the Best Choice
For most people, generics work perfectly. But there are exceptions. Some drugs have a narrow therapeutic index - meaning even tiny changes in blood levels can cause big problems. These include:
- Levothyroxine (for thyroid disease)
- Warfarin (a blood thinner)
- Phenytoin and carbamazepine (for seizures)
For these, the American Epilepsy Society found that 12.7% of patients switching between different generic versions had breakthrough seizures - compared to just 4.3% who stayed on one consistent version. That’s why some neurologists prefer to keep patients on the same brand or even the same generic manufacturer. It’s not that generics are unsafe - it’s that consistency matters more when the margin for error is slim.
Also, complex delivery systems can be tricky. Generic inhalers, like the ones for asthma, sometimes don’t deliver the powder the same way. The FDA issued a warning in 2020 about some generic Advair Diskus copies having inconsistent dispersion. Topical creams and patches can behave differently too. If you’re using a patch for pain or hormones, and you notice it’s not working like before, talk to your doctor.

Inactive Ingredients: The Hidden Factor
While the active ingredient is identical, the rest of the pill isn’t. Fillers, dyes, preservatives, and binders can vary. These don’t affect how the drug works - but they can affect how your body reacts.
Some people are sensitive to certain dyes. Red dye #40, yellow #5, or FD&C blue #1 can cause rashes or stomach upset in rare cases. If you’ve ever had an allergic reaction to a pill, it’s likely not the medicine - it’s the coloring. That’s why FDA rules require generic labels to list inactive ingredients. But here’s the problem: many of those labels are hard to read, and pharmacies don’t always give you a full list.
A 2022 University of Michigan study found only 37% of generic drug labels clearly listed potential allergens. If you have known allergies - to lactose, gluten, or dyes - ask your pharmacist for the full ingredient list. You can also check the FDA’s DailyMed database online for exact formulations.
What to Do When You Switch
Switching doesn’t mean you’re done paying attention. Here’s what to do next:
- Check your pill - Take a picture of the new one. Note the color, shape, and imprint. Keep it in your phone. That way, if you get a different generic next time, you’ll know.
- Monitor for changes - For the first two weeks, keep a simple log. Are you feeling the same? Any new dizziness, nausea, or mood shifts? For diabetes, track your fasting blood sugar. For blood thinners, watch your INR levels. For depression or anxiety, use the PHQ-9 or GAD-7 apps to score your symptoms weekly.
- Call your doctor if something’s off - If you notice reduced effectiveness or new side effects, don’t assume it’s "all in your head." It might be the filler. Or it might be a different generic from a new manufacturer. Your doctor can request the specific brand or manufacturer.
- Stick with one generic - If you find a generic that works well, ask your pharmacist to keep ordering it from the same company. Some people do better with Teva’s version than Sandoz’s, even if both are labeled "metoprolol."
Why Doctors Push Generics - And Why You Should Too
Most doctors don’t push generics because they’re cheap. They push them because they work - and because people stick with them.
Kaiser Permanente found that diabetic patients on generic metformin had a 78% adherence rate. Those on the brand-name version? Only 63%. Why? Because cost matters. When a medication is affordable, people take it. When it’s not, they skip doses, split pills, or stop altogether. That’s far more dangerous than any theoretical risk from a generic.
And the numbers back it up. Medicare beneficiaries save an average of $1,268 a year by using generics. GoodRx data shows 92% of patients who switched reported being satisfied. One woman in Phoenix told ABC15 she was skipping her cholesterol pill because it cost $350 a month. After switching to the generic - $15 - she started taking it every day. Her cholesterol dropped. Her anxiety went away.
What’s Changing in 2025
The rules are getting better. Starting in 2024, Medicare must cover all FDA-approved generics without prior authorization. In 2025, the FDA will require clearer labeling of allergens on all generic drug packaging. And more complex generics are coming - things like orally disintegrating tablets, nasal sprays, and transdermal patches that were once too hard to copy.
The biggest news? Biosimilars. These are the next generation of generics for complex biologic drugs like Humira. The first Humira biosimilar hit the U.S. market in January 2024. They’re not exact copies - they’re highly similar - but they still cut costs by 15% to 30%. This is the future: more options, lower prices, and the same results.
Final Thought: Trust the Science, Not the Label
Switching from brand to generic isn’t a gamble. It’s a smart, science-backed choice. The FDA doesn’t approve generics lightly. They test them. They monitor them. They track them.
Yes, you might get a pill that looks weird. Yes, you might feel unsure. But if you’re taking a common medication - for blood pressure, cholesterol, diabetes, depression, or thyroid - the odds are overwhelmingly in your favor. The medicine inside is the same. The outcome will be too.
Don’t let the color scare you. Ask questions. Keep track. Stay consistent. And save your money - because your health shouldn’t cost a fortune.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove they work the same way in the body through bioequivalence testing. Studies show that 88% of the time, generics perform identically to brand-name drugs in clinical outcomes.
Why do generic pills look different from brand-name ones?
Trademark laws require generic drugs to look different from the brand-name version. That means the color, shape, size, or imprint on the pill must change. This has nothing to do with effectiveness - it’s purely a legal requirement to avoid confusion. The active ingredient inside is still identical.
Can generic drugs cause new side effects?
Rarely. Side effects from generics are usually due to differences in inactive ingredients - like dyes, fillers, or preservatives - not the active drug. If you’re sensitive to certain additives, you might experience a rash, stomach upset, or allergic reaction. Always check the ingredient list if you have known allergies. If you notice new or worsening side effects after switching, contact your doctor.
Are there drugs I shouldn’t switch to generic?
Yes. Drugs with a narrow therapeutic index - like levothyroxine, warfarin, phenytoin, and carbamazepine - require very precise blood levels. Small changes can affect safety or effectiveness. For these, your doctor may recommend staying on one specific brand or generic manufacturer. Always discuss this with your provider before switching.
How can I tell if my generic is working the same way?
Monitor your symptoms and any measurable health markers. For blood pressure or cholesterol, track your readings. For diabetes, check your fasting blood sugar. For thyroid issues, ask for a TSH test a few weeks after switching. For mood or anxiety, use standardized tools like PHQ-9 or GAD-7. Keep a simple journal for two weeks. If you notice a change, talk to your doctor - it might be the generic, or it might be something else.
Why do some people say their generic doesn’t work as well?
Some people experience real differences, especially with drugs that have a narrow therapeutic index. In those cases, switching between different generic manufacturers can cause slight variations in absorption. Also, psychological factors - like believing a brand-name drug is better - can influence perception. But in most cases, the drug is working the same. If you’re concerned, ask your pharmacist to stick with the same manufacturer, or request your doctor to specify "do not substitute."
Can I ask my pharmacist to give me the brand-name drug instead?
Yes, but you’ll likely pay more. Your doctor can write "Dispense as Written" or "Do Not Substitute" on the prescription. In some cases, insurance may still cover the brand if you can prove medical necessity - for example, if you had a bad reaction to a generic. But without that, you’ll usually pay the full cash price.
Are generic drugs made in the same facilities as brand-name drugs?
Sometimes. Many brand-name companies also make generic versions of their own drugs. Other times, generics are made in the same factories, just under a different label. The FDA inspects all manufacturing sites - whether for brand or generic - using the same standards. Quality control is not determined by the name on the bottle.
What should I do if I think my generic is causing a problem?
First, don’t stop taking it without talking to your doctor. Then, note what changed - when it started, what symptoms you’re having, and whether they improved after switching back. Contact your pharmacist to confirm which manufacturer made your pill. Report any serious side effects to the FDA’s MedWatch program at 1-800-FDA-1088. You can also use GoodRx’s free tool to compare your generic to the brand and see if others have reported similar issues.
Will my insurance always cover the generic?
Almost always. Most insurance plans, including Medicare Part D, require you to try the generic first. They often charge much less for it - sometimes $0 or $5. If you’re being charged more for the generic, double-check your plan’s formulary. Starting in 2024, Medicare must cover all FDA-approved generics without prior authorization, making access even easier.
Next steps: If you’re considering a switch, ask your pharmacist for a printout of the inactive ingredients. Write down the appearance of your new pill. Set a reminder to check in with yourself after two weeks. And if you’re worried - talk to your doctor. You’re not being difficult. You’re being informed.






Matthew Ingersoll
December 27, 2025 AT 03:31Generic metformin saved me $280 a month. I didn’t notice any difference in how I felt. The pill looks weird, but my HbA1c hasn’t budged. Stop overthinking the color.
carissa projo
December 27, 2025 AT 07:31It’s easy to fear what you don’t recognize - a white oval instead of a blue diamond - but the science doesn’t care about aesthetics. What matters is your body’s response. If you’re stable, if your numbers are good, if you’re not waking up dizzy or nauseous - then trust that the molecule inside is doing exactly what it’s supposed to. The pill’s appearance is just its uniform. The work happens beneath the surface.
And if you’ve ever felt guilty for wanting the brand because it ‘felt safer’ - that’s not weakness. It’s conditioning. We’ve been sold the illusion that more expensive means better. But medicine isn’t fashion. It’s biology. And biology doesn’t care about logos.
So if you’re switching, take a picture. Note the imprint. Track your symptoms. Be gentle with yourself as you adjust. You’re not being paranoid. You’re being responsible. And that’s something to be proud of.
josue robert figueroa salazar
December 28, 2025 AT 19:37david jackson
December 30, 2025 AT 03:20Let me tell you about the time I switched from brand-name Lexapro to the generic escitalopram - I swear to god, I felt like my brain had been dipped in lukewarm oatmeal for three weeks. No anxiety, no depression, just… flat. Like someone turned down the volume on my entire emotional spectrum. I thought I was going crazy. I tracked my PHQ-9 scores religiously. I cried over a pill bottle. I called my psychiatrist three times. And then - and this is the kicker - I realized the generic was made by a different manufacturer. The first one was Teva. The second was Mylan. I asked my pharmacist to stick with Teva. And suddenly - the fog lifted. The colors came back. I could laugh again. So yes, generics are the same. But not all generics are the same. And if you’re one of those people who feels ‘off’ after switching - don’t just accept it. Find the one that works. Your mind deserves that much.
Jody Kennedy
December 31, 2025 AT 21:50Switching to generic blood pressure meds was the best financial decision I ever made. I used to skip doses because I couldn’t afford it. Now I take it every day without thinking. My BP is better than it’s been in 10 years. And yeah, the pill looks like a tiny chalk eraser - but I don’t care. I’m alive. I’m stable. That’s what matters.
Also - side note - if you’re worried about dyes? Check the label. I’m allergic to Red #40 and I once got a rash from a generic ibuprofen. Now I only take the ones that say ‘dye-free.’ It’s easy to find. Just ask your pharmacist. They’ll give you the sheet.
christian ebongue
January 1, 2026 AT 18:42jesse chen
January 2, 2026 AT 23:40I just want to say - thank you for writing this. So many people are scared to switch, and I get it - the pills look different, the packaging is confusing, and no one ever explains it well. But this? This is clear. Real. Helpful. I showed this to my mom, who’s on warfarin, and she finally felt okay about the switch. She was terrified. Now she takes her pills without a second thought. You made a difference.
Also - I’ve been on the same generic metoprolol from Teva for three years. My heart rate is steady. My anxiety is down. I even asked my pharmacist to order it every time. I don’t care if it’s not the brand. I care that it works. And that’s all that matters.
Joanne Smith
January 3, 2026 AT 01:26Let’s be real - the real issue isn’t the drug. It’s the pharmacy’s inability to communicate. I got a new generic last month. No one told me it was different. I didn’t know the imprint changed. I thought I was getting a new prescription. I panicked. Turned out it was just Sandoz instead of Teva. I had to Google the pill ID. That shouldn’t be on me. Pharmacists need to do better. A quick sticky note on the bag - ‘Same med, new look’ - would save so many people stress.
Also - I switched from brand to generic for my thyroid med. TSH went from 2.1 to 4.8. I felt like a zombie. I went back to the brand. TSH back to normal. So yes - sometimes, it’s not just the manufacturer. It’s the formulation. And your doctor should know that.
Prasanthi Kontemukkala
January 3, 2026 AT 15:00In India, we’ve been using generics for decades - not because we can’t afford brands, but because we trust the science. My father has been on generic lisinopril for 12 years. His blood pressure is perfect. He never had a problem. The pill is white and round. It looks nothing like the one he started with. But his health? Unchanged. We don’t fear the look. We trust the process. Maybe we’ve just seen enough to know: the medicine doesn’t wear a logo. It wears your health.
Alex Ragen
January 4, 2026 AT 14:10It’s fascinating - and frankly, tragic - that we’ve constructed an entire cultural mythology around pharmaceutical branding, as if the placebo effect of a corporate logo can somehow enhance molecular bioavailability. We are, in essence, paying for aesthetic privilege. The FDA’s bioequivalence standards are not suggestions - they are rigorous, peer-reviewed, statistically validated benchmarks. Yet, we allow marketing departments to weaponize our cognitive dissonance, convincing us that a blue capsule imbued with the aura of ‘trust’ is somehow pharmacologically superior to a white one bearing the sterile anonymity of a manufacturer’s code. This is not medicine. This is consumerist fetishism, masquerading as healthcare.
And yet - we still cling to the myth. We fear the unknown shape. We distrust the unfamiliar imprint. We forget: the body does not recognize logos. It recognizes molecules. And molecules - unlike brand equity - do not lie.