When a doctor prescribes a biologic for rheumatoid arthritis, Crohn’s disease, or cancer, they’re using a complex, living-cell-derived medicine. These drugs have changed lives - but they’re expensive. Enter biosimilars: nearly identical versions that cost 15% to 30% less. Yet, despite being approved by the FDA since 2015 and making up nearly a quarter of the U.S. biologics market by value, biosimilars are still misunderstood by many providers. Why? Because they’re not generics. And confusing them with generics is a dangerous mistake.
Biosimilars Aren’t Generics - And That Matters
Generics are chemically synthesized copies of small-molecule drugs. Think metformin or lisinopril. Their structure is simple, predictable, and easy to replicate. If two generics have the same active ingredient, they’re considered interchangeable by default. Biosimilars are different. They’re made from living cells - yeast, bacteria, or mammalian cells - and their structure is far more complex. Even tiny changes in the manufacturing process can alter how the drug behaves in the body. That’s why the FDA requires biosimilars to undergo extensive testing: analytical studies, animal tests, and at least one clinical trial to prove they’re "highly similar" with no clinically meaningful differences in safety, purity, or potency compared to the original biologic. A 2016 survey of 102 U.S. physicians found that only 38% could correctly define a biosimilar. Even worse, 63% couldn’t tell the difference between biosimilars and generics. That gap hasn’t closed much. In 2021, a study of 500 pharmacists showed only 22% felt "very confident" explaining biosimilar differences to patients.What the FDA Actually Requires
The FDA’s approval process for biosimilars is rigorous. It’s not enough to show the molecule looks similar under a microscope. Developers must prove:- Identical amino acid sequence to the reference product
- Comparable structure and function using advanced analytical tools
- No clinically meaningful differences in immunogenicity - the body’s immune response
- Equivalent pharmacokinetics (how the body absorbs and processes the drug)
- Equivalent pharmacodynamics (how the drug affects the body)
Interchangeable vs. Biosimilar: A Critical Distinction
Not all biosimilars are created equal. There’s a special category called "interchangeable." An interchangeable biosimilar has passed an additional set of studies proving that switching back and forth between it and the reference product doesn’t increase risk or reduce effectiveness. This matters because in 42 U.S. states, pharmacists can substitute an interchangeable biosimilar without telling the prescriber - just like they do with generics. As of November 2023, only 10 of the 46 approved biosimilars in the U.S. have interchangeable status. The rest require a prescriber to specifically order them. Many providers don’t realize this distinction. A 2022 survey found that 78% of U.S. hospitals struggled with EHR systems that couldn’t clearly flag whether a drug was interchangeable or just biosimilar. That leads to billing errors, confusion, and sometimes, patients getting the wrong drug.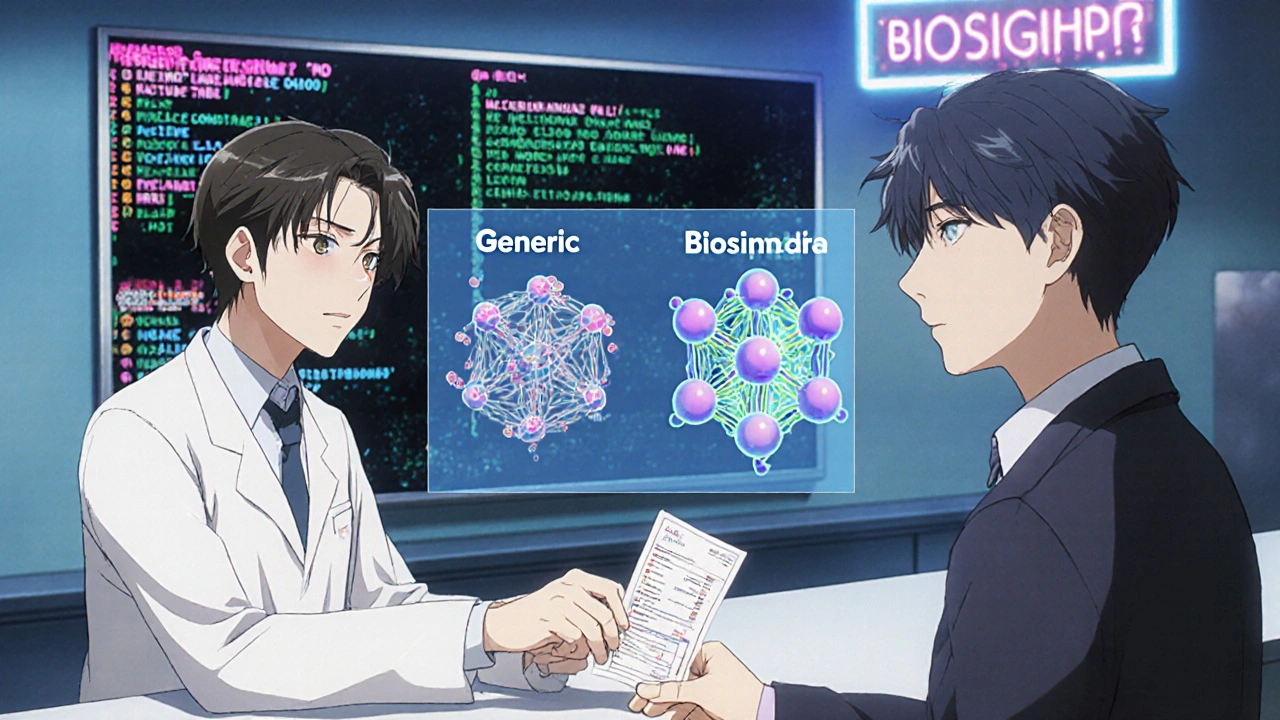
Why Adoption Varies So Much by Specialty
Biosimilar use isn’t evenly spread. Rheumatologists lead the way, with 68% using them routinely. Oncologists follow at 52%. But endocrinologists? Only 29%. Why the gap? It’s not about access. Insulin biosimilars have been available since 2015. It’s about education - and fear. Rheumatologists see the data. They’ve watched patients switch from Humira to its biosimilar and experience the same pain relief, same side effects, same lab results. Oncologists have seen similar results with trastuzumab and bevacizumab biosimilars. But endocrinologists treat diabetes - a chronic, lifelong condition. They’re cautious. A patient’s blood sugar might be stable for months. Switching insulin brands feels risky, even if the science says it’s safe. A 2022 ArthritisPower survey found that 34% of rheumatology patients felt confused when switched to a biosimilar - not because the drug didn’t work, but because their provider didn’t explain why.Education Works - Here’s How
The good news? When providers get proper education, confidence soars. In a 2017 study of oncology staff, provider confidence in biosimilar efficacy jumped from 40.1% to 92% after 12 training sessions over four months. At UCSF Medical Center, pharmacist-led education cut prescribing hesitancy from 58% to 12% in six months. At the University of Pittsburgh, a three-phase program - foundational knowledge, specialty-specific training, and ongoing support - led to 89% provider confidence within six months. What works in these programs?- Clear comparisons: Biosimilar vs. generic - side by side
- Real-world data: Show actual patient outcomes, not just trial results
- EHR walkthroughs: How to document biosimilar use correctly
- Role-playing: Practicing how to explain biosimilars to patients
Where the System Still Fails
Even with great education, the system fights back. Electronic health records often don’t distinguish between biosimilars and reference products. Nurses report spending extra time manually entering data in Epic or Cerner. Pharmacists say insurance forms don’t have a dropdown for "biosimilar," forcing them to type it in - which leads to claim denials. States have different rules. In 12 states, pharmacists must notify the prescriber immediately when substituting an interchangeable biosimilar. In 24, they have seven days. In six, no notification is required. Providers don’t know which rules apply in their state - or even in their own hospital. And then there’s the cost. Medicare Part B pays for biologics based on average sales price. Biosimilars are cheaper, but the reimbursement system doesn’t always reflect that savings. Some hospitals still get paid the same amount whether they use the original or the biosimilar - so there’s no financial incentive to switch.What You Need to Know Right Now
If you’re a provider - whether you’re a doctor, nurse, pharmacist, or pharmacist - here’s what you need to do:- Know the difference: Biosimilars are not generics. They’re complex, living drugs with rigorous testing.
- Check interchangeability: Is the biosimilar labeled "interchangeable"? If not, it can’t be substituted without your order.
- Understand extrapolation: If a biosimilar works for one condition, it’s scientifically valid to use it for others with the same target - even if not directly studied.
- Use the FDA’s free resources: Their Teaching Resource Guide is updated annually and includes real-world case studies.
- Ask for help: Talk to your hospital’s clinical pharmacist. They’re often the best source of up-to-date biosimilar knowledge.
What’s Next
By 2025, the Association of American Medical Colleges plans to have biosimilar education in 95% of medical school curricula. The FDA is updating its guide in 2024 to include more real-world evidence - because providers are asking for it. The market is growing fast. Global biosimilar sales hit $12.3 billion in 2022. In Europe, 80% of eligible biologics are now biosimilars. In the U.S., it’s just 32%. But that gap is closing - if providers get the right education. The goal isn’t just to save money. It’s to get life-changing treatments to more patients. That only happens when providers understand what biosimilars really are - and how to use them confidently.Are biosimilars the same as generics?
No. Generics are chemically identical copies of small-molecule drugs and require only bioequivalence testing. Biosimilars are complex, large-molecule drugs made from living cells. They must undergo extensive analytical, non-clinical, and clinical testing to prove they’re "highly similar" with no clinically meaningful differences from the original biologic. The FDA requires much more evidence for biosimilars than for generics.
Can a biosimilar be automatically substituted like a generic?
Only if it’s designated as "interchangeable" by the FDA. Interchangeable biosimilars have passed additional studies proving that switching between them and the reference product doesn’t affect safety or effectiveness. In 42 U.S. states, pharmacists can substitute an interchangeable biosimilar without a prescriber’s permission - just like with generics. But if the biosimilar is not interchangeable, the prescriber must specifically order it.
Why do some providers hesitate to use biosimilars for conditions not directly studied?
This is called extrapolation - using data from one condition to approve a biosimilar for others with the same biological target. While the FDA requires strong scientific justification and has approved it for decades, many providers remain skeptical. Surveys show 57% express concern. But the evidence is solid: 37 clinical trials involving over 12,500 patients support biosimilar use in rheumatoid arthritis, and the same mechanism applies to psoriasis and other conditions. The American College of Rheumatology strongly recommends it.
How do biosimilars affect patient outcomes?
Real-world data shows no difference. Studies from Europe and the U.S. show that patients switching from originator biologics to biosimilars experience the same clinical outcomes - including disease control, hospitalization rates, and side effects. In oncology, response rates to biosimilar trastuzumab are identical to the original. In rheumatology, pain and mobility scores remain unchanged. The key is proper education: providers who understand biosimilars report 92% confidence in their efficacy, compared to just 40% among those who haven’t been trained.
What’s the biggest barrier to biosimilar adoption?
The biggest barrier is knowledge - not access or cost. While biosimilars are cheaper and available, many providers don’t understand how they work, how to document them, or how to explain them to patients. EHR systems often can’t distinguish between biosimilars and reference products, leading to billing errors. A 2022 survey found 78% of U.S. hospitals had EHR documentation challenges. Education programs that include hands-on training, pharmacist involvement, and real-world data are the most effective way to overcome this.







Rebekah Kryger
November 16, 2025 AT 23:27Biosimilars aren't generics? Newsflash: neither is a Tesla a Toyota. Just because both have four wheels doesn't mean you can swap the engine and expect the same ride. The FDA’s ‘highly similar’ standard is a legal loophole dressed up as science. If you can’t prove exact equivalence, why are we calling it ‘interchangeable’? This isn't innovation - it's cost-cutting with a fancy label.
Victoria Short
November 17, 2025 AT 21:18Yeah but like... I just want my insulin to work. Don't care what it's called.
Eric Gregorich
November 18, 2025 AT 04:34Let’s peel back the onion here - this isn’t just about pharmacology, it’s about the existential crisis of modern medicine. We’ve outsourced trust to regulatory agencies and corporate labs, and now we’re surprised when patients balk at a new vial labeled ‘similar but not the same.’ The biologic was a miracle - a living molecule, a whisper of human biology made into medicine. A biosimilar? It’s a photocopy of a photocopy, printed on cheap paper by a machine that doesn’t understand the original’s soul. We’re treating life-saving drugs like commodities, and the consequence isn’t just clinical - it’s spiritual. When we reduce wonder to efficiency, what are we really saving? The system? Or our own capacity to believe in healing?
Koltin Hammer
November 19, 2025 AT 12:53Look, I get the fear. I’m an endocrinologist in rural Iowa. My patients are on insulin for 40 years. They know their brand. They trust it. You toss in a biosimilar and suddenly they’re Googling ‘is this gonna kill me?’ - not because the science is shaky, but because the system never explained it right. I had a lady cry because her new pen looked different. Not because it didn’t work - because nobody sat down and said, ‘This is the same, just cheaper.’ We treat patients like data points, not humans. The FDA’s guide? It’s gold. But if your EHR doesn’t even label it right, what’s the point? We need more than education - we need a cultural shift in how we talk about medicine. Not ‘biosimilar’ - say ‘equivalent.’ Not ‘extrapolated’ - say ‘it works the same way.’ Language matters. And so does empathy.
Phil Best
November 19, 2025 AT 19:52Ohhhhhh so now we’re pretending biosimilars are ‘safe’ because some PhDs in a lab in Maryland said so? Let me guess - the same people who told us Vioxx was fine? Or that thalidomide was ‘not teratogenic in animals’? This isn’t science - it’s corporate theater. And don’t even get me started on ‘interchangeable.’ So now pharmacists are playing doctor? Next thing you know, your asthma inhaler gets swapped for a ‘similar’ one made in a basement in Bangalore. This is how you turn healthcare into a Walmart aisle. And the worst part? They’re selling this as ‘progress.’ Progress? Nah. It’s just capitalism with a stethoscope.
Parv Trivedi
November 19, 2025 AT 23:48I come from India, where biosimilars have been used for over a decade. We call them ‘affordable miracles.’ In our hospitals, patients with rheumatoid arthritis who couldn’t afford Humira now walk without pain. Yes, education is needed - but fear should not block access. The data is clear: outcomes are the same. Let’s not let bureaucracy silence hope. A child in rural Bihar deserves the same chance as a child in Boston. Biosimilars aren’t a compromise - they’re justice.
Willie Randle
November 20, 2025 AT 07:07There’s a critical grammatical error in the original post: ‘whether you’re a doctor, nurse, pharmacist, or pharmacist.’ The redundancy is unintentional but misleading - it implies pharmacists are a subset of pharmacists, which is logically incoherent. Beyond that, the content is excellent. The FDA’s Teaching Resource Guide is underutilized because it’s buried in a PDF on a .gov site. It needs a redesign: mobile-friendly, scannable, with embedded videos. Also, ‘extrapolation’ should be replaced with ‘mechanism-based approval’ - it’s clearer and less intimidating. Precision in language isn’t pedantry - it’s patient safety.
Connor Moizer
November 21, 2025 AT 01:13You’re all overthinking this. If the damn thing works, use it. My clinic switched to the Humira biosimilar last year - 120 patients, zero adverse events, $300K saved. The EHR still messes up the coding? Fix it. The pharmacist doesn’t know the difference? Train them. The patient’s scared? Tell them: ‘This is the same drug, just less expensive.’ Done. Stop making it a philosophy lecture. This isn’t quantum physics - it’s medicine. And if you’re still hesitating because you didn’t read the 12-module guide? That’s your problem, not the drug’s.
kanishetti anusha
November 22, 2025 AT 08:46When I first saw a biosimilar insulin in my hospital, I thought it was a mistake. But I watched a diabetic patient’s HbA1c stay stable for 18 months after switching. No complications. No complaints. I started asking my colleagues: ‘Why are we scared?’ It’s not about the science - it’s about fear of change. We need more stories like this - not just data. Real people. Real results. And we need to stop calling it ‘biosimilar.’ Call it ‘equivalent biologic.’ It’s less scary. And more honest.
roy bradfield
November 24, 2025 AT 05:59This whole biosimilar thing is a controlled demolition of the pharmaceutical industry’s profit model. The FDA? They’re in bed with Big Pharma. The ‘rigorous testing’? It’s theater. The real data? Buried in proprietary databases. The ‘interchangeable’ label? A marketing trick to get pharmacists to swap drugs without oversight. And don’t tell me about the 37 trials - who funded them? Who owns the patents? The same companies that made the original biologic. This isn’t progress - it’s a legal shell game. And the patients? They’re the pawns. One day, someone’s going to get sick because a biosimilar had a tiny impurity that slipped through. And then we’ll have our scandal. Mark my words.
Patrick Merk
November 25, 2025 AT 20:36Back home in Ireland, we’ve been using biosimilars since 2010. No chaos. No mass adverse events. Just cheaper drugs and happier budgets. The key? Transparency. Every prescription says ‘biosimilar’ - and the pharmacist explains it to the patient in plain language. No jargon. No fear-mongering. We treat patients like adults. And guess what? They trust us more. The FDA’s guide is brilliant - but it needs a Dublin-style rewrite: short, warm, and human. No ‘extrapolation’ - say ‘it works for this too because the target is the same.’ Simple. Clear. Effective.
Liam Dunne
November 27, 2025 AT 12:03My hospital ran a pilot last year: switched all new rheumatoid arthritis patients to the Humira biosimilar. We tracked lab values, pain scores, and hospital visits for a year. Results? Statistically identical. Zero difference. The only thing that changed? Our pharmacy budget went from ‘bleeding red’ to ‘actually sustainable.’ The real issue isn’t the science - it’s the inertia. Providers are busy. EHRs are garbage. Insurance forms are medieval. Fix the system, not the science. And for God’s sake, update the dropdown menus.